
A Proactive Approach to Improved Blood Safety
No matter where you are located, increased travel, climate change, evolving pathogens, emerging pandemics and the need to advance healthcare practices have put global blood safety at the forefront of industry focus.
With all this change, it’s time to take a proactive approach to protecting patients from the potential dangers of donated blood. The Mirasol PRT system can help you do it by reducing the pathogen load of a broad range of disease-causing viruses, bacteria and parasites. The system also inactivates residual white blood cells found in blood components,1,2,3 which may help to reduce transfusion reactions in patients.

Powerful, Proven and So Much Potential
The Mirasol PRT system is the only PRT system shown in a human clinical trial to reduce the transfusion-transmitted infection (TTI) of a disease, reducing TTI of malaria by 87%.4
The Right Balance for Better Blood
We believe better blood safety should provide the right balance of efficacy and ease of use while maintaining the quality of blood components for patients who need them. The Mirasol PRT system is a simple system that has the power to deliver better patient care, reducing the risks associated with the lifesaving gift of blood.
The Mirasol PRT System
You know better than anyone—these are challenging times. The Mirasol PRT system allows you to meet the needs of your business by optimizing the balance of cost, efficacy and safety of your blood products.5,6
Safety
Mirasol PRT provides an added layer of protection against tested pathogens (HIV, HBV, HCV) during the window period7 as well as against untested/emerging pathogens. It uses a combination of riboflavin (vitamin B2), a nontoxic and nonmutagenic substance,8 and UV light.
Mirasol reduces the pathogen load of a broad range of disease-causing viruses, bacteria and parasites and inactivates residual white blood cells in blood products.1,2,3 For example:
READ MORE
CLOSE

Learn more
Prof. Raymond P. Goodrich and Dr. Pavel Trakhtman discuss blood safety during the COVID-19 pandemic and the use of COVID-19 convalescent plasma.
Access Now
Article : Keil S, et al. Vox Sang. 2020;115(6):495-501.
Access Now
Article : Keil SD, et al. Transfusion. 2015;55(7):1736-1744.
Access Now
Poster: Ojea A, et al. Vox Sang. 2020;115(suppl s1):224-225. P-433.
Access Now
Simplicity
Mirasol requires minimum handling and few procedure steps and allows for immediate product release.15
Learn More
CLOSE
Learn more
Interviews with Dr. Ashley Duits, Director, Red Cross Blood Bank Foundation Curaçao; Dr. John-John Schnog, Hematologist, Medical Oncologist, Curaçao Biomedical & Health Research Institute; Dr. Ginette Ecury, Pediatrician, Neonatologist, Curaçao Medical Center.
Access Now
Affordability
The routine use of Mirasol may replace bacterial testing,11 gamma irradiation18 and cytomegalovirus (CMV) screening (if leukoreduction is used)1,2,3 and may reduce the expired product discard rate (based on a 7-day shelf life for platelets in PAS).15 Operational practices may vary by center.
Learn More CLOSE
There is no compound removal step17 in the Mirasol process. That means you can expect these benefits as compared with other pathogen reduction technologies:
- Minimal platelet loss, less than < 5% for both apheresis- and WB-derived buffy coat platelets19,20,21
- Avoid extra platelet agitators
- Avoid the potential need to manage two different inventories of blood products (one for the compound removal step and one for storage)

Learn more
Peace of Mind
With Mirasol, there are no known contraindications for use1,2,3 and no acute/long-term effects for patients, including pediatrics,22,23 because riboflavin and its photoproducts are nontoxic and nonmutagenic.8
For processing staff, there are no additional health hazard risks or precautions required for product handling24 because there is no need to remove the chemical compound.17
Learn more
Interviews with Dr. Ashley Duits, Director, Red Cross Blood Bank Foundation Curaçao; Dr. John-John Schnog, Hematologist, Medical Oncologist, Curaçao Biomedical & Health Research Institute; Dr. Ginette Ecury, Pediatrician, Neonatologist, Curaçao Medical Center.
Access Now
Dr A.J. Duits, Head of the Department of Immunology of Curaçao Biomedical & Health Research Institute, talks about his experience with Mirasol PRT during different epidemics and COVID-19 pandemic, and Shawn D. Keil, Scientific Affairs, Terumo Blood and Cell Technologies, explains the performance of the Mirasol PRT system against non-enveloped viruses.
Access Now
Trusted Partner
The Mirasol PRT system is offered by Terumo Blood and Cell Technologies, a partner you can count on. We provide a comprehensive portfolio and dedicated expertise in all steps of blood transfusion and therapeutic apheresis.
Once the technology is in place, we continue to serve you with:
- Education and training
- Technical support
- Clinical and scientific support
- Customer support
- User groups and professional networks
How It Works
The Right Path to Advancing Blood Safety: Safety, Simplicity, Affordability, Peace of Mind
The Mirasol PRT system uses a combination of nontoxic, nonmutagenic riboflavin (vitamin B2) and a specific spectrum of ultraviolet (UV) light to inactivate viruses, bacteria, parasites and white blood cells that may be present in collected blood products.

The Mirasol PRT system consists of three main components:
- A disposable kit — includes an illumination/storage bag and sterile riboflavin solution
- The Mirasol Illuminator — provides UV light and agitation for the Mirasol treatment
- Mirasol Manager software — integrates and manages data reporting and storage
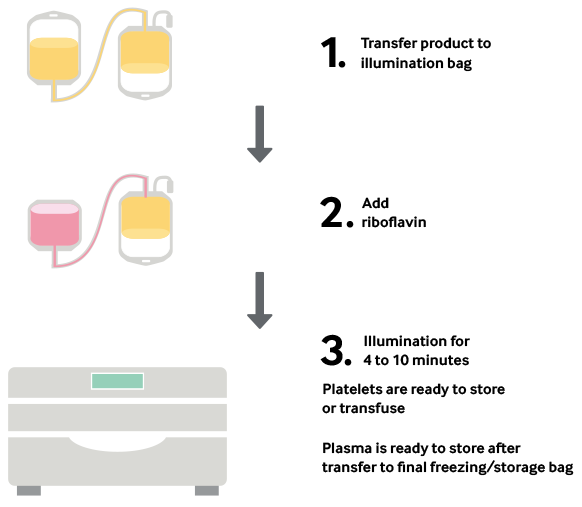
During treatment, a blood product is mixed with the riboflavin solution and placed into the Illuminator, where it is exposed to UV light. There is no need to remove riboflavin or its photoproducts; after illumination, the treated products are ready for transfusion or placement into storage.
Mode of Action
The Mirasol PRT system causes irreversible changes to the RNA and DNA of viruses, bacteria, parasites and white blood cells, preventing them from replicating and causing disease.
Riboflavin + UV light = irreversible inactivation of pathogens and white blood cellsVideo: Mirasol Mode of Action
The Mirasol PRT system uses a combination of riboflavin (vitamin B2), a non-toxic, non-mutagenic compound, and a specific spectrum of ultraviolet (UV) light to inactivate viruses, bacteria, parasites and white blood cells that may be present in collected blood products.

The Process
Platelets, Plasma and Now Whole Blood in Four Simple Steps
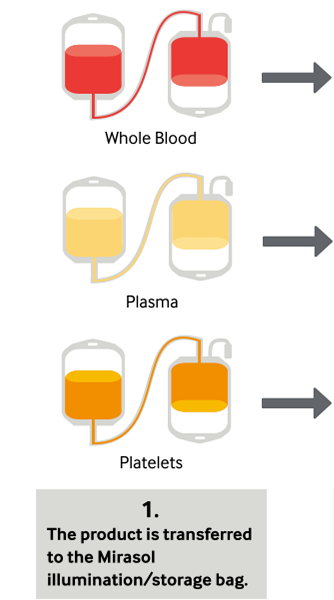
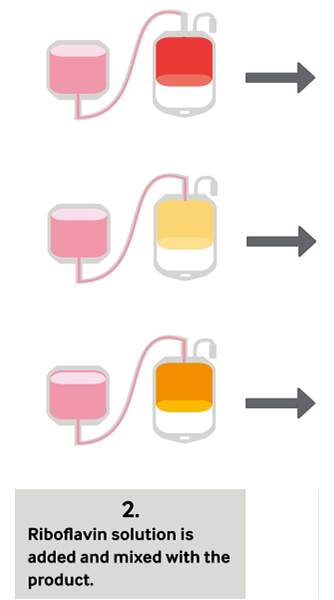
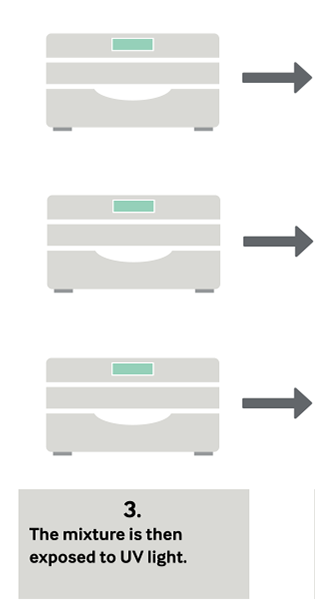
Clinical Studies With Mirasol-Treated Platelets
Clinical Study: PREPAReS
The Pathogen Reduction Evaluation & Predictive Analytical Rating Score (PREPAReS) clinical trial is the most comprehensive one to date demonstrating the non-inferiority of Mirasol-treated platelets.
Webinar
Clinical Experience With Mirasol-Treated Platelets, presented by Dr. Jeffrey McCullough, an international leader in research and practice of transfusion medicine and blood safety.
Watch On-Demand
Fact Sheet
PREPAReS clinical study supports the safety and efficacy of Mirasol-treated platelets.
Download
Clinical Data Overview
Routine use data and clinical trials support the safety and effectiveness of Mirasol-treated blood products.
Download
Clinical Study: IPTAS
The Italian Platelet Technology Assessment Study (IPTAS) was designed as two independent, randomized controlled trials (RCTs) conducted simultaneously in six hematological centers in Italy to assess the effectiveness and safety of pathogen-reduced versus non-pathogen-reduced platelets. Although conclusions on non-inferiority could not be drawn due to low statistical power, the study provides additional information on the safety and efficacy of pathogen-reduced platelets treated with two commercial pathogen-reduction technologies.
Study Results Article:
Rebulla P, et al. Clinical effectiveness of platelets in additive solution treated with two commercial pathogen-reduction technologies. Transfusion. 2017. 57(5):1171-1183.
Download
References: Click here CLOSE
1Terumo Blood and Cell Technologies. Mirasol Platelet Disposable Kit for Treatment in Platelet Additive Solution Instructions for Use. December 2016. Part no. 777710-058.2Terumo Blood and Cell Technologies. Mirasol Plasma Disposable Kit Instructions for Use. Part no. 777712-011.
3Terumo Blood and Cell Technologies. Mirasol Whole Blood Disposable Kit Instructions for Use. Part no. 777714-014.
4Allain JP, Owusu-Ofori AK, Assennato SM, Marschner S, Goodrich RP, Owusu-Ofori S. Effect of Plasmodium inactivation in whole blood on the incidence of blood transfusion-transmitted malaria in endemic regions: the African Investigation of the Mirasol System (AIMS) randomised controlled trial. Lancet. 2016;387(10029):1753-1761.
5Custer B, Agapova M, Martinez RH. The cost-effectiveness of pathogen reduction technology as assessed using a multiple risk reduction model. Transfusion. 2010;50(11):2461-2473.
6Agapova M, Lachert E, Brojer E, Letowska M, Grabarczyk P, Custer B, et al. Introducing pathogen reduction technology in Poland: a cost-utility analysis. Transfus Med Hemother. 2015;42(3):158-165.
7Kleinman SH, Lelie N, Busch MP. Infectivity of human immunodeficiency virus-1, hepatitis C virus, and hepatitis B virus and risk of transmission by transfusion. Transfusion. 2009;49(11):2454-2489.
8Piper J, et al. Evaluation of genotoxicity and acute toxicity risks associated with a riboflavin based pathogen inactivation process. Presented at: 22nd Annual Meeting of the American College of Toxicology; November 2001; Washington, D.C.
9Keil SD, Ragan I, Yonemura S, Hartson L, Dart NK, Bowen R. Inactivation of severe acute respiratory syndrome coronavirus 2 in plasma and platelet products using a riboflavin and ultraviolet light‐based photochemical treatment. Vox Sang. 2020;115(6):495-501.
10Ragan I, Hartson L, Pidcoke H, Bowen R, Goodrich R. Pathogen reduction of SARS-CoV-2 virus in plasma and whole blood using riboflavin and UV light. PLOS ONE. 2020;15(5):e0233947.
11Ruane PH, Edrich R, Gampp D, Keil SD, Leonard RL, Goodrich RP. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion. 2004;44:877-885.
12Marschner S, Goodrich R. Pathogen reduction technology treatment of platelets, plasma and whole blood using riboflavin and UV light. Transfus Med Hemother. 2011;38(1):8-18.
13Owada T, Kaneko M, Matsumoto C, et al. Establishment of culture systems for genotypes 3 and 4 hepatitis E virus (HEV) obtained from human blood and application of HEV inactivation using a pathogen reduction technology system. Transfusion. 2014;54(11):2820-2827.
14Cerus. INTERCEPT Brochure. 2016. Part no. MKT-EN 00147-01 v3.0.
15Jimenez-Marco T, Garcia-Recio M, Girona-Llobera E. Our experience in riboflavin and ultraviolet light pathogen reduction technology for platelets: from platelet production to patient care. Transfusion. 2018;58(8);1881-1889.
16Sutton S, Smith D. A report on the evaluation of two pathogen reduction/inactivation systems at the Western Province Blood Transfusion Service. Presented at: 34th South Africa National Blood Transfusion Congress; Sun City; 2017.
17Boehnke C, Reuter U, Flach U, Schuh-Hofer S, Einhaupl KM, Arnold G. High-dose riboflavin treatment is efficacious in migraine prophylaxis: an open study in a tertiary care centre. Eur J Neurol. 2004;11(7):475-477.
18Fast LD, DiLeone G, Marschner S, et al. Inactivation of human white blood cells in platelet products after pathogen reduction technology treatment in comparison to gamma irradiation. Transfusion. 2011;51(7):1397-1404.
19Marciniak-Bielak D. A feasibility study of OrbiSac buffy coat platelet concentrates treated with the Mirasol PRT system. Vox Sang. 2011;101(suppl 1):187.
20Van der Meer PF, Couture C, Hervig T, et al. Experiences with semi-routine production of riboflavin and UV-B pathogen-inactivated platelet concentrates in three blood centres. Vox Sang. 2017;112(1):9-17.
21Coene GC, De Schrijver E, Sabot B, Dereen D, Reynaerts I, Vandekerckhove P. In vitro and in vivo evaluation of Mirasol pathogen reduction technology treated platelets stored in SSP+. Vox Sanq. 2011;101(suppl 1):188.
22Piotrowski D, Przybylska-Baluta Z, Jimenez-Marco T, et al. Passive haemovigilance of blood components treated with a riboflavin-based pathogen reduction technology. Blood Transfus. 2018;16(4):348-351.
23Olsen JH, Hertz H, Kruger Kjaer S, Bautz A, Mellemkjaer L, Boice JD. Childhood leukemia following phototherapy for neonatal hyperbilirubinemia (Denmark). Cancer Causes Control. 1996;7(4):411-414.
24Terumo Blood and Cell Technologies. Mirasol System―Riboflavin Solution Globally Harmonized System of Classification and Labeling of Chemicals (GHS)―Safety Data Sheet (SDS). Part no. 777701-519A.
