Several vascular access options are available for sickle cell disease patients receiving transfusion therapy.
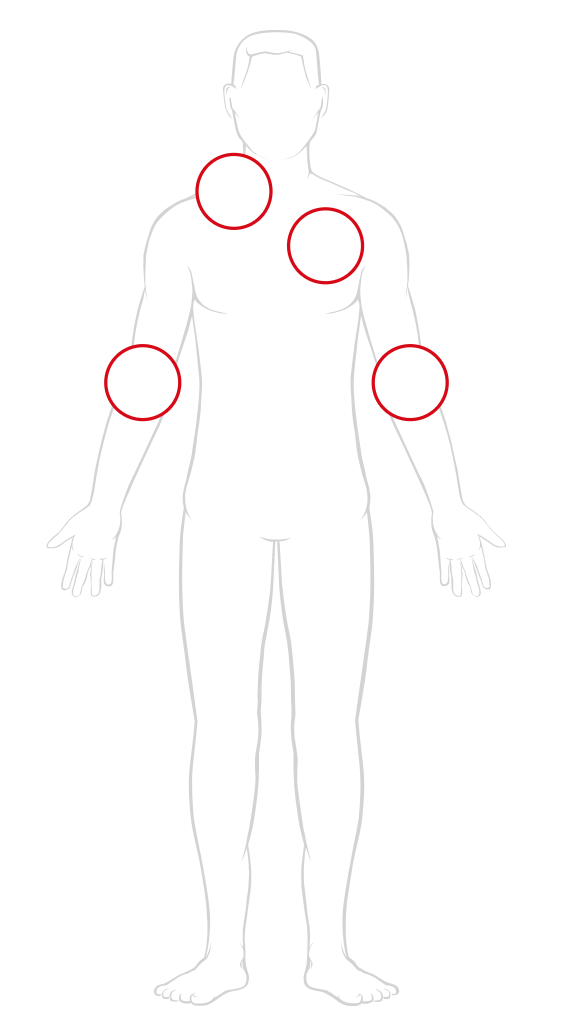
Click to learn about vascular access options.
A central venous catheter (CVC) can be temporary or permanent.
- A long, thin, hollow plastic tube called a "catheter" or "line" is placed in a central vein. A central vein is a large vein located in the neck, upper chest or groin.
- This kind of access requires placement by specialists.
- Typically, this type of access is associated with automated exchange transfusion.
*Vascular access varies from patient to patient and is often determined by vascular access specialists.
Ask your doctor about your vascular access options.
An implanted port is a more permanent solution.
- The implanted port is a device that is placed under the skin into a central vein.
- A central vein is a large vein located in the neck, upper chest and inner thigh.
- This kind of access requires placement by specialists.
- Typically this type of access is associated with exchange transfusion.
*Vascular access varies from patient to patient and is often determined by vascular access specialists.
Ask your doctor about your vascular access options.
Peripheral venous access is temporary and uses a needle in one or both arms.
- Complications are consistently less frequent and less severe than with a central venous catheter (CVC).12
- These rare complications may include risk of infection, discomfort, thrombosis (blood clots), vein infiltration (catheter goes into, or comes out of your vein), and sclerosis of the veins (hardening of the blood vessels).17
- The clinician may use an ultrasound device to help properly place the line.
Additional types of vascular access:
- Arteriovenous (AV) fistula
- AV graft
- Implanted port
*Venous access varies from patient to patient and is often determined by vascular access specialists. Peripheral access may not be an option for all patients.
Ask your doctor about your vascular access options.
A central venous catheter (CVC) can be temporary or permanent.
- A long, thin, hollow plastic tube called a "catheter" or "line" is placed in a central vein. A central vein is a large vein located in the neck, upper chest or groin.
- This kind of access requires placement by specialists, and the patient must undergo local anesthesia.
- Potentially serious complications have been associated with central venous catheters.2
*Venous access varies from patient to patient and is often determined by vascular access specialists.
Ask your doctor about your vascular access options.
An implanted port is a more permanent solution.
- The implanted port is a device that is placed under the skin into a central vein.
- A central vein is a large vein located in the neck, upper chest and inner thigh.
- This kind of access requires placement by specialists.
- Typically this type of access is associated with exchange transfusion.
*Vascular access varies from patient to patient and is often determined by vascular access specialists.
Ask your doctor about your vascular access options.
Peripheral venous access is temporary and uses a needle in one or both arms.
- Complications are consistently less frequent and less severe than with a central venous catheter (CVC).12
- These rare complications may include risk of infection, discomfort, thrombosis (blood clots), vein infiltration (catheter goes into, or comes out of your vein), and sclerosis of the veins (hardening of the blood vessels).17
- The clinician may use an ultrasound device to help properly place the line.
Additional types of vascular access:
- Arteriovenous (AV) fistula
- AV graft
- Implanted port
*Venous access varies from patient to patient and is often determined by vascular access specialists. Peripheral access may not be an option for all patients.
Ask your doctor about your vascular access options.
Your clinician may determine that one of these other types of vascular access is right for you:
- Arteriovenous (AV) fistula
- AV graft
Potential complications of vascular access
- Peripheral vascular access
- Complications are less frequent and less severe than with a CVC.12
- These rare complications may include risk of infection, discomfort, thrombosis (blood clots), vein infiltration (the catheter moves, causing fluid to enter the tissues surrounding the vein), and sclerosis of the vein (hardening of the blood vessel).17
- Central venous catheter (CVC)
- Infection and thrombosis (blood clots) are the most frequent complications with long-term use of a CVC.18
- Implanted port
- Because the port is implanted completely under the skin, the risk of infection is lower than with a CVC.18
- The device may be dislodged, requiring it to be re-implanted.
1World Health Organization (WHO). Fifty-ninth world health assembly. Sickle-cell anaemia: report by the Secretariat. https://who.int. Published April 24, 2006. Accessed June 1, 2020.
2American Society of Hematology. Understanding the Impact of Sickle Cell Disease. https://www.hematology.org/education/patients/anemia/sickle-cell-disease. Accessed November 5, 2020.
3Danielson CF. The role of red blood cell exchange transfusion in the treatment and prevention of complications of sickle cell disease. Ther Apher. 2002;6(1):24-31.
4Centers for Disease Control and Prevention. Sickle Cell Disease. Data & Statistics on Sickle Cell Disease. https://www.cdc.gov/ncbddd/sicklecell/data.html#:~:text=SCD%20affects%20approximately%20100%2C000%20Americans,sickle%20cell%20trait%20(SCT). Accessed June 1, 2020.
5Tsitsikas DA, Sirigireddy B, Nzouakou R, et al. Safety, tolerability, and outcomes of regular automated red cell exchange transfusion in the management of sickle cell disease. J Clin Apher. 2016;31(6):545-550.
6Dedeken L, Le PQ, Rozen L, et al. Automated red blood cell exchange compared to manual exchange transfusion for children with sickle cell disease is cost-effective and reduces iron overload. Transfusion. 2018;58(6):1356-1362.
7Kuo K, Ward R, Kaya B, Howard J, Telfer P. A comparison of chronic manual and automated red blood cell exchange transfusion in sickle cell disease patients. Br J Haematol. 2015;170(3):425-428.
8National Institute for Health and Care Excellence (NICE). Spectra Optia for automatic red blood cell exchange in patients with sickle cell disease. https://www.nice.org.uk/guidance/mtg28/chapter/5-Cost-considerations. Accessed November 2020.
9Singer T, Quirolo K, Nishi K, Hackney-Stephens E, Evans C, Vichinsky E. Erythrocytapheresis for chronically transfused children with sickle cell disease: an effective method for maintaining a low HbS level and reducing iron overload. J Clin Apher. 1999;14(3):122-125.
10Duclos C, Merlin E, Paillard C, et al. Long-term red blood cell exchange in children with sickle cell disease: manual or automatic? Transfus Apher Sci. 2013;48(2):219-222.
11Howard J. The role of blood transfusion in sickle cell disease. ISBT Science Series. 2013;8:225-228.
12Barth D, Sanchez A, Thomsen A, et al. Peripheral vascular access for therapeutic plasma exchange: a practical approach to increased utilization and selecting the most appropriate vascular access. J Clin Apher. 2020;35(3):178-187.
13Adams DM, Schultz WH, Ware RE, Kinney TR. Erythrocytapheresis can reduce iron overload and prevent the need for chelation therapy in chronically transfused pediatric patients. J Pediatr Hematol Oncol. 1996;18(1):46-50.
14Fasano RM, Leong T, Kaushal M, Sagiv E, Luban NL, Meier ER. Effectiveness of red blood cell exchange, partial manual exchange, and simple transfusion concurrently with iron chelation therapy in reducing iron overload in chronically transfused sickle cell anemia patients. Transfusion. 2016;56(7):1707-1715.
15Al-Salem AH. Medical and Surgical Complications of Sickle Cell Anemia. Springer; 2016.
16Cobianchi C, Fafoutis D, Roig J, Dierick K, Comasolivas N. Measuring health-related quality of life in individuals with sickle cell disease undergoing automated red blood cell exchange. Poster presented at: Sickle Cell Disease Association of America Annual Meeting: October 2018; Baltimore, MD.
17Barth D, Nemec RM, Cho DD, et al. The practical integration of a hybrid model of ultrasound-guided peripheral venous access in a large apheresis center. J Clin Apher. 2020; 35(4):328-334.
18Crookston KP. Therapeutic Apheresis: a Physician’s Handbook. 5th ed. Bethesda, MD: AABB/ASFA; 2017.
19AABB. Circular of Information for the Use of Human Blood and Blood Components. Bethesda, MD: AABB; 2017.
20European Directorate for the Quality of Medicines & HealthCare (EDQM). Guide to the Preparation, Use and Quality Assurance of Blood Components. 20th ed. Strasbourg, France: EDQM Council of Europe; 2020.